
Since the amount of 5.65 M added is not asked for, there is no need to solve for it. Here is the second way to solve this problem: Where x is volume of 5.65 M HCl that is added Here is the first way to solve this problem: Problem #4: What volume of 4.50 M HCl can be made by mixing 5.65 M HCl with 250.0 mL of 3.55 M HCl? The solution to this problem assumes that the volumes are additive. Problem #3: How many milliliters of 5.0 M copper(II) sulfate solution must be added to 160 mL of water to achieve a 0.30 M copper(II) sulfate solution? Please note how I use the molarity unit, mol/L, in the calculation rather than the molarity symbol, M. What molarity would the potassium nitrate solution need to be if you were to use only 2.5 L of it? Problem #2: You need to make 10.0 L of 1.2 M KNO 3. Remember to keep the volume units consistent. Note that 1000 mL was used rather than 1.0 L. Problem #1: If you dilute 175 mL of a 1.6 M solution of LiCl to 1.0 L, determine the new concentration of the solution. Ten Examples Problems #11 - 25 Problems #26 - 35 Return to Solutions Menu
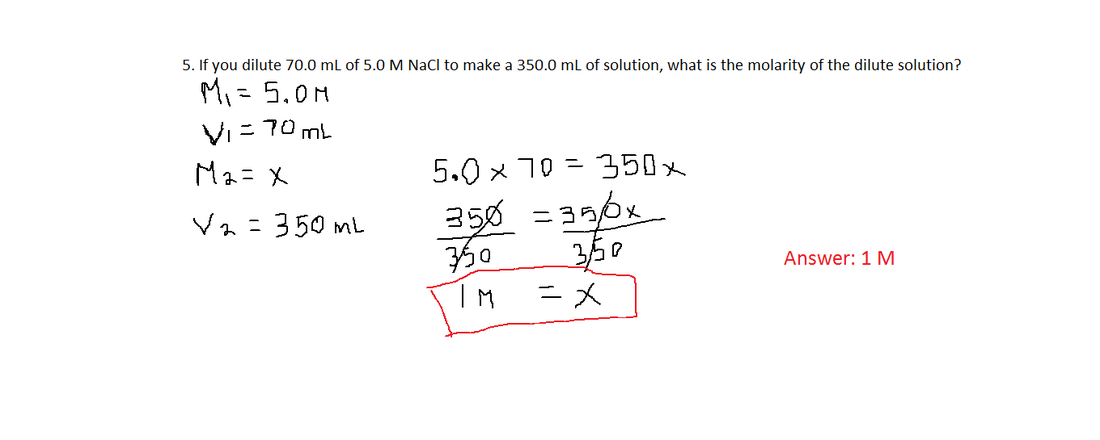

ChemTeam: Dilution Problems #1-10 Dilution Problems


 0 kommentar(er)
0 kommentar(er)
